Top Head
-

- by Lee, Jeong-Hwan Nov 3, 2025 06:08am
Minister of Health and Welfare Eun-kyeong Jeong expressed support for introducing international nonproprietary name (INN) prescribing and expanding substitution dispensing by pharmacists for medicines facing supply instability, as they are national policy tasks. However, she stressed the need to build social consensus given strong opposition from the medical community. Minister Jeong also expressed her intent to establish a direction through research services on how to monitor out-of-stock drugs and set criteria and definitions to resolve the unstable supply of pharmaceuticals. During the National Assembly Health and Welfare Committee audit on the 30th, Minister Jeong responded so to Rep. Jong-tae Jang Democratic Party of Korea) on his inquiries. Rep. Jang had proposed a bill mandating limited INN prescribing for pharmaceuticals with unstable supply. Minister Jeong appealed to the validity of limited INN prescribing, citing factors such as the excessively low domestic substitution rate compared to advanced countries overseas. Rep Jang’s view is that legislating INN prescribing and promoting substitution can simultaneously achieve three goals: resolving the unstable supply of medicines, reducing the National Health Insurance Service's drug expenditure burden, and strengthening patients' choice of medicines. Rep. Jang explained, “In the U.S., nearly all states legally allow pharmacists to substitute with equivalent generic drugs. Some states have even introduced automatic substitution. They also adopt a system where the National Health Insurance prioritizes generic drugs for reimbursement and imposes additional coinsurance rates when original brand drugs are prescribed. As a result, generic prescriptions account for 91% of prescriptions.” He added, “In Japan, pharmacists can freely substitute drugs unless specifically prohibited by a ‘do not substitute’ label. Since last year, they've even introduced a surcharge for requesting brand-name drugs when an original prescription is specified. Pharmacies and medical institutions receive incentives based on their generic drug prescription rates, resulting in an 82% generic prescription rate.” Rep. Jang stated, “The domestic situation, with generic substitution at only 1.5%, is woefully low compared to the US at 90% and Japan and the UK at 80%. The Lee Jae-myung administration's core pledges are resolving essential drug shortages, promoting generic substitution, optimizing drug expenditure efficiency, and solving the rebate issue. The amendment to the Pharmaceutical Affairs Act mandating INN prescriptions is a proven policy to achieve this. What does the Minister think about this?“ Minister Jeong replied, “It is included in the national agenda to review INN prescribing and utilize substitution for drugs with unstable supply. We will consider introducing INN prescribing for essential medicines with unstable supply first.” She added, “Given the significant opposition and disagreement in the medical community, we will proceed through social consensus and dialogue. We also need to discuss how to monitor and define drugs with unstable supply, so we will conduct a short-term research project and discuss the results with MFDS.”
Headline
- Opinion
- [Desk View] Unsettling relief from pharma tariff agreement
- by Chon, Seung-Hyun Nov 3, 2025 06:09am
- Analysis suggests that the burden of tariffs on the pharmaceutical sector has been significantly alleviated following the settlement of the US-Korea tariff negotiations, which had dragged on for about five months. According to the agreement, the Most Favored Nation (MFN) designation will be granted to synthetic new drugs and biological new drugs (biosimilars/biobetters) in the pharmaceutical sector. Generic drugs and natural resources not produced in the United States will be subject to zero tariffs. The pharmaceutical and biotech industries express clear relief. Concerns over pharmaceutical tariffs emerged after President Donald Trump stated on his social media platform, Truth Social, in September that "100% tariff would be imposed on all branded drugs and patented drugs from companies not building manufacturing plants in the U.S. starting the following month." The Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA) welcomed the agreement, stating, "We greatly welcome the settlement of the Korea-U.S. tariff negotiations." They added, "Securing MFN designation along with maintaining zero tariffs for generic drugs ensures trade conditions that are not unfavorable compared to other major countries. We expect this to positively impact the strengthening of our pharmaceutical and bio-industry's global competitiveness." The KoreaBio Association also welcomed the agreement, stating, "With the tariff negotiation settlement, South Korea is not disadvantaged compared to competitors like Europe and Japan when exporting pharmaceuticals to the U.S., and we expect a significant reduction in trade uncertainties with the U.S." In fact, the expectation was that imposing tariffs would not have a significant impact on the overall domestic pharmaceutical industry, as the scale of Korean pharmaceutical and biotech companies' exports to the U.S. is small. Only a few companies, such as Celltrion, Samsung Biologics, Samsung Bioepis, SK Biopharmaceuticals, Daewoong Pharmaceutical, and Green Cross Corp, are actively pursuing entry into the U.S. market. The majority of domestic pharmaceutical and biotech companies have no experience entering the U.S. market. The fact is that the industry as a whole did not show strong interest because the pharmaceutical tariff risk was seen as 'someone else's problem' for most pharmaceutical and biotech companies. According to the Ministry of Food and Drug Safety (MFDS), the U.S. export value of domestically produced pharmaceuticals in Korea last year was $1.49117 billion (approximately KRW 2 trillion), accounting for only about 1% of the total U.S. export value of $127.8 billion. This is less than 5% of the $36.6 billion in automobile exports. Pharmaceutical exports to the U.S. are also dominated by Samsung Biologics and Celltrion. Samsung Biologics recorded U.S. export sales of KRW 1.1741 trillion last year through Contract Development and Manufacturing Organization (CDMO) services for biopharmaceuticals. Celltrion's biopharmaceutical sales in the North American market last year amounted to KRW 1.0453 trillion. Domestic pharmaceutical and biotech companies have released a total of 40 new drugs since SK Chemicals' Sunpla in 1999, up to Medytox's Nuviju in September this year. However, products that have entered the U.S. market are limited to LG Chem's Factive, Dong-A ST's Sivextro, Hanmi Pharmaceutical's Rolontis, and Yuhan Corporation's Leclaza. Most domestically developed new drugs that enter the U.S. do not have large export sales. Leclaza is gradually increasing sales after recent U.S. approval, but this does not count toward Korea's export performance, as the product is manufactured locally by its partner, Janssen, for sale in the U.S. This is the background why the pharmaceutical industry welcomed the US-Korea tariff negotiation, but did not achieve consensus across the entire industry. It is why they seem to be in vain. The settled negotiation is welcome, but it offers little immediate tangible benefit. In contrast to the vigorous success of domestic manufacturing, such as K-Beauty and K-Food, in the U.S. market, the performance report for K-Bio in the U.S. is still at a nascent stage. The majority of domestic pharmaceutical companies are still largely dependent on the domestic market. However, the recent increase in U.S. exports is encouraging. In 2014, the U.S. export value of domestic pharmaceuticals was $120.57 million, accounting for only 5.0% of the total export value of $2.40349 billion. Last year, the U.S. share of total pharmaceutical exports was 16.1%, an increase of more than threefold from 10 years ago. While the total pharmaceutical export volume last year increased by 3.9 times compared to 10 years ago, the U.S. export value increased by 12.4 times during the same period. The increase in U.S. exports of finished pharmaceutical products is a positive development. In 2014, API exports to the U.S. were $88.53 million, more than double the finished pharmaceutical product exports of $32.04 million. Last year, finished pharmaceutical products accounted for $1.29899 billion of U.S. pharmaceutical exports, exceeding API exports of $192.19 million by more than five times. In 2014, the U.S. ranked 6th among destination countries for pharmaceutical exports, but it jumped to 1st place last year. The U.S. export value of finished pharmaceutical products last year expanded by 40.5 times compared to 10 years ago, which is in contrast to the 2.2-fold increase in API U.S. export value over the past 10 years. This shows that finished pharmaceutical products incorporating domestic companies' R&D technology are gradually accelerating their penetration into the U.S. market, thus slowly but incrementally expanding their presence. Recently, not only biosimilars but also domestically developed botulinum toxin products and blood products are actively pursuing entry into the U.S. market. The success of new drug out-licensing among biotech companies is spreading, and there are increasingly noticeable cases of global pharmaceutical companies launching new drugs using K-Bio technology. We hope for a time when the R&D achievements of domestic pharmaceutical and biotech companies are used as wild cards on the global stage, leveraged in negotiations, and when successful tariff negotiations translate into tangible benefits.
- Company
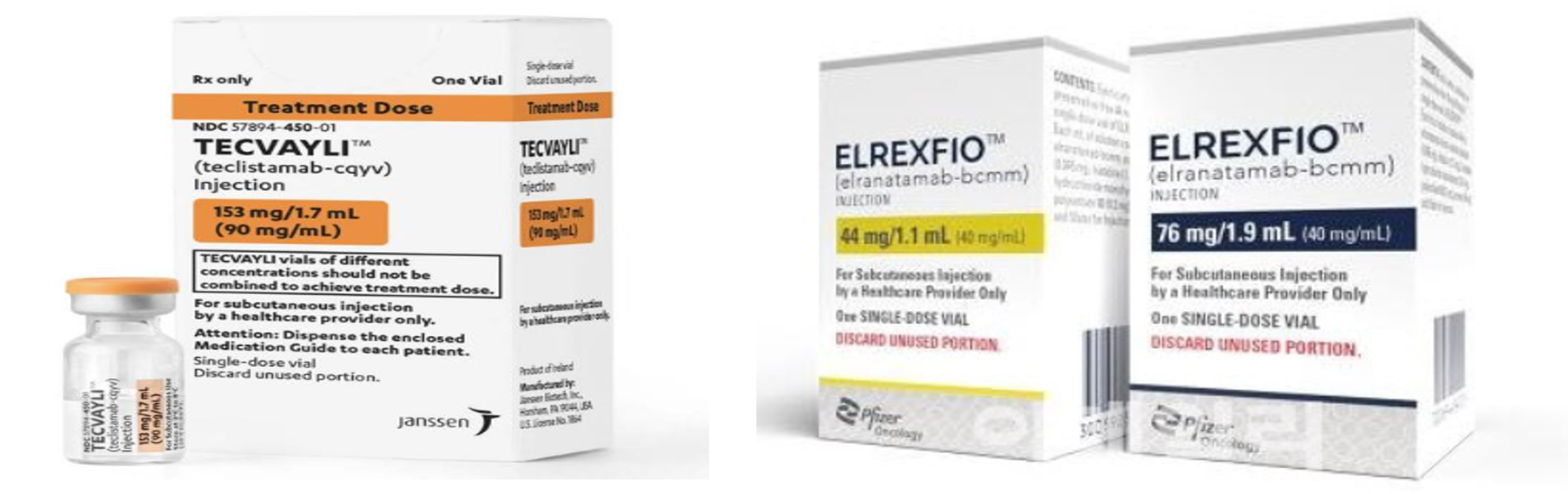
- Bispecific antibodies pass reimbursement hurdle
- by Son, Hyung Min Nov 3, 2025 06:09am
- Major bispecific antibody therapies targeting hematologic cancers have successively cleared the first stage of reimbursement. With patients who have failed prior therapies desperately needing new options, attention is focused on whether these new drugs with innovative-mechanism will secure insurance reimbursement. According to industry sources on November 3, the Health Insurance Review & Assessment Service (HIRA) recently held its 8th Cancer Disease Review Committee (CDRC). At the meeting, reimbursement criteria were established for two hematologic bispecific antibodies: Janssen's 'Tecvayli (teclistamab)' and 'Pfizer's Elrexfio (elranatamab).' Multiple myeloma is a blood cancer characterized by the proliferation of abnormal plasma cells, which are created in the bone marrow, throughout the body. A relatively large number of treatments have emerged for this cancer type, with new drugs being approved for up to fifth-line treatment and beyond. Besides bispecific antibodies, various CAR-T new drugs like 'Kymriah (tisagenlecleucel)' and 'Yescarta (axicabtagene ciloleucel)' also target this disease. Due to exiting alternative like CAR-T had led to the assessment that reimbursement for bispecific antibodies would not be easy. However, limitations of CAR-T therapies include complex manufacturing processes, the time delay of over a month until administration, and the requirement for patients to maintain a certain health condition. Unlike these, bispecific antibodies are off-the-shelf anti-cancer drugs that work by simultaneously recognizing cancer cells and T-cells, activating the T-cells to indirectly eliminate the cancer cells. BCMA is a protein expressed on the surface of B cells, and CD3 is expressed on the surface of T-cells. Elrexfio and Tecvayli have a novel mechanism that induces myeloma cell death by targeting both. Elrexfio demonstrated efficacy in the MagnetisMM-3 Phase 2 study in patients who had failed three or more prior therapies. In the trial, Elrexfio showed an Objective Response Rate (ORR) of 61%, and the rate of maintained response at 15 months was confirmed to be 71%. The median Overall Survival (OS) for the Elrexfio group was 24.6 months, and the median Progression-Free Survival (PFS) was 17.2 months. Tecvayli was administered to 165 patients in the MajesTEC-1 Phase 2 study, resulting in an ORR of 63%, with a stringent Complete Response (sCR) of 32.7%, a Complete Response (CR) of 6.7%, and a Very Good Partial Response (VGPR) of 19.4%. The median time to response was 1.2 months, and the duration of response was reported to be 18.4 months. Bispecific antibodies for B-cell lymphoma also await to be reviewed Discussions on reimbursement for bispecific antibodies are also progressing in relapsed/refractory Diffuse Large B-cell Lymphoma (DLBCL), another type of hematologic cancer. Among these, AbbVie's 'Epkinly (epcoritamab)' passed the CDRC in July, after its reimbursement criteria were established. DLBCL is the most common B-cell lymphoma, accounting for about 40% of non-Hodgkin lymphomas, and has an aggressive nature with rapid progression. The failure rate after first-line treatment reaches about 15%, and even patients who achieve CR have a relapse rate of 25% within 18 months. Although existing CAR-T therapies are reimbursed and utilized, their application to elderly patients is limited due to treatment initiation delays and neurotoxicity (ICANS). Thus, the demand for new treatments still exists. Epkinly was evaluated in the EPCORE NHL-1 Phase 1/2 study, involving 167 patients with CD20-positive relapsed or refractory DLBCL who had received two or more prior lines of therapy. The results showed an ORR of 62%, a CR of 39%, and a median Duration of Response (DOR) of 15.5 months. Attention is also focused on the re-attempt for Roche's bispecific antibody, 'Columvi (glofitamab).' Columvi was submitted to the CDRC in December last year and July this year but failed to establish reimbursement criteria. Columvi was submitted concurrently with Epkinly in December last year; however, Epkinly succeeded in passing the CDRC hurdle in June on its second attempt. Since Columvi has expanded its indication to second-line DLBCL treatment in the U.S. and Europe, Roche may be considering a simultaneous reimbursement application for both second- and third-line settings. Columvi is a treatment with a maximum duration of 12 cycles, approximately 8 months, and has a defined end of treatment. In clinical trials, Columvi showed a CR of 40%, an ORR of 52%, and a median DOR of 26.9 months for patients who achieved CR. It also recorded a 67% CR maintenance rate at 18 months.
- Company
- Olympus to redefine BPH treatment landscape with iTind
- by Hwang, byoung woo Nov 3, 2025 06:09am
- Olympus Korea is moving to reshape the urology market with its benign prostatic hyperplasia (BPH) treatment device, “iTind.” Leveraging the advantages of its non-resection, shape-memory alloy structure, the company is highlighting key features such as sexual function preservation and rapid recovery as it pushes to expand market penetration. Olympus Korea held a press conference on the 31st to outline strategies to broaden adoption at domestic clinics and improve patient accessibility following the recognition of its new health technology. Olympus Korea strengthens urology portfolio Olympus Korea already possesses a diagnostic and therapeutic portfolio for various diseases, including cancer, through medical endoscopes, laparoscopes, and surgical equipment. Its core areas are gastroenterology, respiratory, and urology, while also providing diagnostic and therapeutic solutions in otolaryngology, surgery, and other fields. In April, Olympus Korea introduced the minimally invasive BPH treatment device ‘iTind’ to the domestic market. iTind is a temporary implantable device made from Nitinol alloy. When inserted into the prostatic urethra in a folded state, it gradually unfolds at the 5 o'clock, 7 o'clock, and 12 o'clock positions in response to body temperature, gently exerting pressure on the prostatic urethra and bladder neck. This process induces localized ischemia and tissue remodeling, widening the urinary channel. The device is removed after 5&8211;7 days via a simple procedure, leaving no foreign material behind. Junsoo Lee, SP Marketing Sub-Unit Leader at Olympus Korea, stated, “iTind is a non-incisional treatment that creates a channel without using energy, thus avoiding tissue damage. It is a minimally invasive option that preserves sexual function while offering rapid recovery.” The first domestic procedure was performed at Kangdong Sacred Heart Hospital in April. The company noted that the simple procedure enables use even at clinic-level facilities and plans gradual site expansion. Jeongsoo Kim, SP Unit Leader at Olympus Korea, added, “Unlike resection surgery, it offers differentiated value by leaving no foreign objects in the body and enabling faster recovery. It will become a new treatment strategy that reduces the burden on both patients and clinicians.” Clinical evidence-based efficacy and safety...strengths of new health technology iTind’s clinical data simultaneously demonstrated long-term efficacy and safety. In a 48-month follow-up study of 81 patients with benign prostatic hyperplasia (BPH), the International Prostate Symptom Score (IPSS) decreased by 45.3%, and maximum urinary flow rate (Qmax) increased by 114.7%. A 12-month follow-up multicenter study (120 patients) confirmed a 54.9% reduction in IPSS and a 106.6% increase in Qmax, with no reports of sexual dysfunction or ejaculation disorders. Naeun Min, UG Marketing Cell Leader at Olympus Korea, stated, “Long-term benefits have been confirmed overseas, and early domestic procedure cases show high patient satisfaction. There is no residual material in the body and no tissue deformation, reducing the burden of repeat procedures.” In this regard, Olympus disclosed on-site feedback from clinicians, noting that patients reported improved urination and a rapid return to daily life after the procedure, indicating high initial satisfaction. iTIND was officially designated as a new health technology by the Ministry of Health and Welfare in May last year and is currently used in 23 countries, including the US, Europe, and Korea. It is also listed in the American Urological Association (AUA) guidelines for benign prostatic hyperplasia (BPH), securing treatment evidence. Confidence in market expansion...“Will enhance patient accessibility to spread treatment value” According to statistics from the Health Insurance Review and Assessment Service, the number of BPH patients in Korea reached 1.61 million in 2024, a roughly 24% increase compared to five years ago (1.3 million in 2019). Of these, 98% are managed with medication, driving rapid growth in demand for the minimally invasive surgical therapies (MIST) category, which bridges the gap between drugs and surgery. Currently, iTind is a non-reimbursed procedure, leading to cost variations between hospitals. This means that even with good technology, cost limitations can create access barriers. Lee noted, “Although non-reimbursed, the cost is roughly half that of existing minimally invasive procedures, improving accessibility. It’s a realistic option for patients delaying surgery or struggling with drug side effects.” In the long term, Olympus Korea is focusing on expanding adoption primarily in clinics and delivering patient-tailored treatment solutions. Lee added, “We will continue education and awareness efforts to position iTind as a low-burden treatment option. Expanding access and delivering the clinical value of iTind will be our key priorities.” Kim concluded, “Olympus Korea is expanding its medical device portfolio beyond cancer treatment to enhance patient quality of life. Beginning with iTind, we will continue delivering innovative solutions in urology and minimally invasive care.”
- Company
- Samsung Bioepis wins second trial in Eylea patent dispute
- by Kim, Jin-Gu Nov 3, 2025 06:08am
- Samsung Bioepis has overturned a prior loss and secured victory in the second-instance trial on invalidating the formulation patent for Eylea (aflibercept). The ruling strengthens expectations that commercial sales of its Eylea biosimilar ‘Afilivu’ may resume. According to industry sources on the 31st, the Patent Court on Oct. 30 ruled in favor of Samsung Bioepis in its lawsuit seeking invalidation of Regeneron’s registered patent. This patent pertains to the composition of Aflibercept. It centers on formulation technology to ensure stable preparation of aflibercept, the main component of Eylea, at high concentrations (40-50mg/mL) as a ‘VEGF antagonist formulation suitable for intravitreal administration’. Samsung Bioepis filed a patent invalidation trial in December 2022. In October last year, the Intellectual Property Trial and Appeal Board (IPTAB) partially dismissed and partially rejected the petition, siding with Regeneron. Following the IPTAB ruling, domestic sales of Samsung Bioepis’ biosimilar Afilivu were suspended in Korea. Based on that decision, Regeneron had filed both a main patent-infringement lawsuit and a preliminary injunction request for sale suspension, and the court granted the injunction in May this year. Samsung Bioepis contested this, filing a lawsuit with the Patent Court to overturn the IPTAB ruling. One year later, the Patent Court reversed the earlier decision and ruled in favor of Samsung Bioepis. As a result, prospects are rising that Afilivu sales may resume. Because the preliminary injunction relied on the IPTAB decision, observers expect the injunction may now be lifted following the Patent Court ruling. The company is also seen gaining a strategic advantage in its broader legal battle with Regeneron. In addition to this invalidation lawsuit, Samsung Bioepis has appealed an earlier loss in the injunction case. The main patent infringement lawsuit is ongoing at the first-instance level.
- Company
- "Kisqali's adjuvant therapy, results from the NATALEE study"
- by Hwang, byoung woo Nov 3, 2025 06:07am
- The 5-year follow-up results of the NATALEE study, unveiled at the European Society for Medical Oncology (ESMO Congress 2025), are drawing significant attention. The data suggest the potential for further widening of the long-term survival curves across a broad patient population, including lower-risk groups such as node-negative patients. This evidence is evaluated as expanding the potential for 'complete remission access' in early breast cancer treatment. Im Seock-Ah of Seoul National University Hospital's Division of Hematology-Medical Oncology assessed that "Patients who are node-negative (N0) or have 1-3 positive nodes often relapse at 5th-7th year," and said, "The preventive effect in this late-relapse patient group will further widen the long-term survival gap." "3-year dosing design, clear evidence for inhibiting early recurrence" Hormone Receptor-positive (HR+) / HER2-negative (HER2-) breast cancer is known to account for approximately 70% of all breast cancers. Furthermore, it is reported that one-third of stage 2 patients and over half of stage 3 patients experience recurrence even after the standard Endocrine Therapy (ET). The NATALEE study is a Phase 3 clinical trial evaluating the long-term recurrence prevention effect of Kisqali (ribociclib, 400mg, 3 weeks on/1 week off) combination therapy compared to non-steroidal aromatase inhibitor (NSAI) monotherapy in 5,101 patients with high-risk stage 2-3 HR+/HER2- early breast cancer. Regarding Kisqali's adjuvant therapy design, Professor Im explained, "The peak of recurrence is most pronounced between 2 and 3 years post-surgery. Continuing treatment for three years to cover this period has clear clinical evidence." She stated, "We have pharmacodynamic data showing that target inhibition is possible even at the 400mg dose, and the suppression of early recurrence is the key factor that widens the gap between the future iDFS and OS curves." The current Hazard Ratio (HR) in the NATALEE study is around 0.8, already indicating a positive outcome. Professor Im assessed that the study is following the same trend as the monarchE study of Verzenio (abemaciclib), which demonstrated improvement in Overall Survival (OS) in its 7-year follow-up analysis. Professor Im emphasized, "As the follow-up of the NATALEE study continues, OS improvement will clearly become visible within 3-4 years." She stressed, "Even an absolute benefit of 2-3% in adjuvant therapy is clinically significant, and the large-scale design of breast cancer studies is intended to secure this statistical power." "Reducing non-reimbursement burden... high economic acceptance" Professor Im also mentioned that patient access to Kisqali in clinical practice is relatively high, although it is currently non-reimbursed. She said, "Novartis is trying to improve price accessibility, and some patients can receive assistance from private insurance," and added, "The perception of it being a 'high-cost new drug' has decreased since it has been used for over seven years in metastatic breast cancer." Professor Im believes that patients view the cost not as spending millions of KRW to live a few more months with metastatic cancer, but as investing a significantly smaller amount to 'live a healthy life forever.' This perspective results in a relatively high rate of patients choosing the drug in clinical practice. However, the patient burden due to the non-reimbursed status still exists. Professor Im also shared her view that simply differentiating between overlapping patient populations when future reimbursement is applied would not be desirable. She emphasized, "Since there are patients who need to switch to Kisqali due to side effects from abemaciclib, or vice versa, the system should allow for flexible patient choice based on individual circumstances." She stressed, "Restrictions like 'you can't use this because an alternative drug exists' ultimately limit patient freedom. The system should be more open based on scientific evidence." Finally, Professor Im concluded by stressing the importance of 'precision medicine' as the future direction for breast cancer treatment, alongside discussions on treatment access. She stated, "Although breast cancer treatment is nearing a cure, there is a need to precisely differentiate the biological differences between the patient group that rapidly progresses early on and the long-term survival group." Professor Im also pointed out, "Genomic testing and biomarker analysis are the answers, but since they are not covered by insurance, the patient burden reaches 80%." He added, "Genuine long-term survival is only possible when medical professionals' scientific judgment is trusted and the system adopts a more flexible approach."
more articles
- [Reporter’s View] The dark side of flashy health campaigns
- Reporter's view | Son, Hyung Min
MOST READ